For my son’s 8th birthday we hosted a Mad Science Party. The kids rotated through stations completing some fun experiments. Most of these experiments can be completed using simple household materials, and I’ve provided some links to some of the harder to find chemicals. I would recommend trying these experiments with school-aged children, 5 and older. If you’re going to attempt these experiments with pre-school aged children, please supervise them very closely. If you’re looking for some easier experiments for little ones, check out Nicole’s post with her favorite experiments for preschoolers. So grab your lab coats and safety goggles, because here we go, my 6 favorite science experiments for big kids.
Diet Coke &
Mentos Explosion
Materials: Mentos Candy, Diet Coke (you don’t need to need name brand soda, the generic diet will work, but it MUST be diet to create the explosion), Geyser Tube Cap (optional, but it’s really cool)
Directions: This is an easy experiment that the kids can do by themselves. Simply drop the Mentos into the diet soda bottle and watch it erupt. The geyser tube is an awesome addition to the experiment because it makes the stream shoot even higher in the air.
Why it works: It’s due to a process called nucleation, where the carbon dioxide in the soda is attracted to the Mentos. That creates so much pressure that the soda goes flying.
Disappearing Iodine Water
Materials: 2 cups of water (about 1/2 way full), Vitamin C, Iodine, Droppers
Directions: Fill two plastic cups 1/2 way full with ordinary room temperature water. Crush up two vitamin C tablets and stir the powder into one of the cups of water. Add approximately a tablespoon of Iodine to the other cup of water. Use your droppers to carefully mix a few drops of iodine water into the vitamin C water. You’ll see that the dark brown Iodine water “disappears” when it hits the vitamin C water. Be careful with this experiment because Iodine will stain clothes (and lab coats). The good news is that some of that vitamin C water will get it out.
Why it works: When Iodine and vitamin C (ascorbic acid) are combined in solution, a chemical reaction takes place. In this chemical reaction, the vitamin C (ascorbic acid) molecule loses electrons, which are transferred to the Iodine molecule.
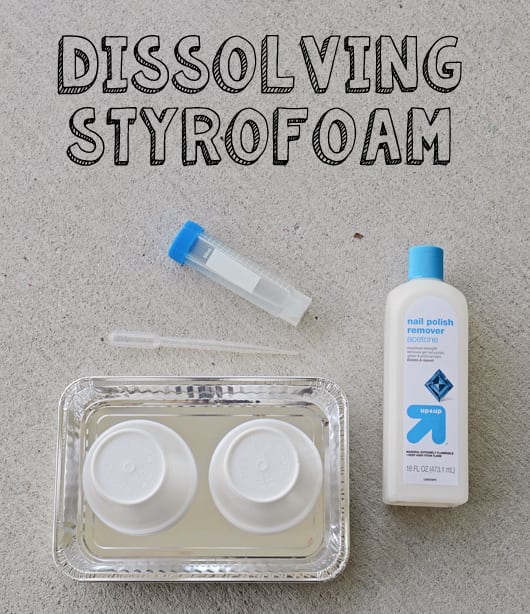
Dissolving Styrofoam
Materials: Styrofoam Cups, Maximum Strength Acetone (nail polish remover), Droppers
Directions: Pour the acetone over the Styrofoam and watch it dissolve (it is dissolving, it is NOT melting).
Why it works: Styrofoam is made up of polystyrene foam. When the polystyrene dissolves in the acetone, the air in the foam is released, causing it to look like you’re dissolving this massive quantity of material into a small volume of liquid.
Exploding Film Canister
Materials: Film Canister, Water, Alka-Seltzer Tablet
Directions: Fill the film canister 3/4 full of water. Add Alka-Seltzer (generic brand works just as well) tablet and close the lid tight. Stand back and watch the lid shoot off into the sky.
Why it works: When the tablet hits water, a chemical reaction takes place very quickly. The bubbles are carbon dioxide gas (CO2) and there’s a lot of it! In the closed canister, the CO2 builds up so much pressure that the lid is forcibly removed from the inside.

Elephant Toothpaste
Materials: Clean & empty soda or water bottle, Yeast, Warm Water, Food Coloring, Dish Soap, 6% Hydrogen Peroxide (you can find this online or at a beauty supply shop)
Directions: In a separate cup mix 1 teaspoon of yeast with 2 tablespoons of warm water. In your soda bottle mix together ½ cup of hydrogen peroxide, a squirt of dish soap and a few drops of food coloring. Swirl the soda bottle to mix slightly. Pour the contents of your yeast & water mixture into the soda bottle and watch your elephant toothpaste erupt.
Why it works: Hydrogen peroxide naturally breaks down into water and oxygen. It is stored in opaque containers to help slow down this process. Yeast speeds up the reaction. Dish soap catches the oxygen and makes bigger bubbles, and the food coloring makes it look cool. The foam and bottle feel warm because the reaction is exothermic–it releases energy as heat.

Inflate A Balloon
Materials: Small Empty Water Bottle, Baking Soda, Vinegar, Balloon, Funnel
Directions: Use the funnel to fill your balloon with 2-3 tablespoons of baking soda. Fill your small water bottle about ½ full with vinegar. Carefully put the top of your balloon over the mouth of the water bottle. Don’t dump the baking soda in until it is secure. Hold your balloon upright and gently shake it so that all the baking soda falls down into the vinegar.
Why it works: The baking soda and the vinegar create an ACID-BASE reaction, and the two chemicals work together to create a gas (carbon dioxide). Gasses need a lot of room to spread out, and the carbon dioxide starts to fill the bottle and then moves into the balloon to inflate it.